Imagine you’re on three different pills for high blood pressure, diabetes, and cholesterol. Your doctor prescribes them separately, but your pharmacist hands you a single pill that does it all. Sounds convenient, right? But what if that pill isn’t legally allowed to replace what your doctor wrote? This is the reality for millions of patients and pharmacists dealing with combination drug substitution-a practice that’s growing faster than the laws around it.
What Exactly Is a Combination Drug?
A combination drug isn’t just two pills in one bottle. It’s a single dosage form-like a tablet, capsule, or injection-that contains two or more active ingredients. Think of ATRIPLA, which combines efavirenz, emtricitabine, and tenofovir into one pill for HIV treatment. Or the combo of pembrolizumab and lenvatinib used together for certain cancers. These aren’t random mixes. They’re carefully designed to work as a unit, often to improve adherence, reduce side effects, or enhance effectiveness. The FDA defines these as combination products when they include drugs, biologics, or devices in one package. That’s important because it means they’re regulated differently than single-drug products. And that difference is where the legal mess begins.Why Can’t Pharmacists Just Swap Them Like Regular Generics?
In most states, pharmacists can legally substitute a brand-name drug with a generic version-same active ingredient, same dose, same effect. That’s generic substitution. But combination drugs break that model. Say your doctor prescribes metformin for diabetes. Your pharmacist wants to switch you to a combo pill that includes metformin and sitagliptin. That’s not generic substitution. That’s therapeutic substitution-replacing one drug with a different one, even if it includes the original. And in most places, that’s not allowed unless the pharmacist has special prescribing authority. The problem? State laws were written decades ago for single-drug generics. They don’t account for pills that contain multiple new chemical entities. The FDA’s own guidance says that if a combination product includes ingredients not listed on the prescription, it can’t be substituted without explicit authorization. Yet, many pharmacists aren’t trained to spot the difference.The Legal Patchwork Across States
There’s no national rule for combination drug substitution. Each state sets its own rules, and they contradict each other. In Texas, pharmacists must follow strict protocols, but the law doesn’t clearly define what counts as a “therapeutically equivalent” combination product. In Alberta, Canada, pharmacists can’t substitute a single drug for a combo unless they have additional prescribing rights. And in California, a 2022 court case (Smith v. CVS Caremark) ruled that adding an extra active ingredient to a prescription without the doctor’s okay is illegal-even if it’s clinically beneficial. Even the definition of “equivalent” is fuzzy. For a single drug, equivalence means matching the exact salt, dose, and release mechanism. But for a combo? Do you match all ingredients? Just the primary one? What if one component is modified-release? What if the combo has a different ratio than the original? There’s no standard.
What Happens When Pharmacists Get It Wrong?
A 2022 survey by the National Community Pharmacists Association found that 68% of independent pharmacists ran into substitution dilemmas at least once a month. Over 40% said they refused to substitute because they weren’t sure if it was legal. Some pharmacists, desperate to cut costs or simplify refills, go too far. They’ve been known to swap a single drug for a combo pill without checking the prescription. Others refuse to substitute even when it’s safe-out of fear of liability. The consequences? Patients get confused. They might take the wrong combo, miss a dose, or experience unexpected side effects. Elderly patients on multiple meds are especially at risk. The American Heart Association warns that inappropriate substitution in cardiovascular combos could lead to adverse events in up to 8% of patients.Who’s Pushing for Change-and Why?
Cost is the biggest driver. Generic drugs make up 90% of prescriptions in the U.S. but only 23% of drug spending. Combination products, though more expensive upfront, can lower long-term costs by improving adherence. One study estimated that expanding substitution for these products could cut medication costs by 15-25% for chronic conditions. The Medicare Part D redesign under the Inflation Reduction Act encourages therapeutic substitution where appropriate. The NHS in England has saved £280 million annually since 2019 by standardizing substitution protocols for heart disease combos. The European Commission has made harmonizing substitution rules for combination medicines a top priority for 2025. But the FDA and experts like Dr. John Smith from the Center for Drug Evaluation and Research warn that without clear rules, cost savings could come at the cost of patient safety. “The current patchwork of state laws creates confusion for pharmacists and potential safety risks,” he told Congress in 2022.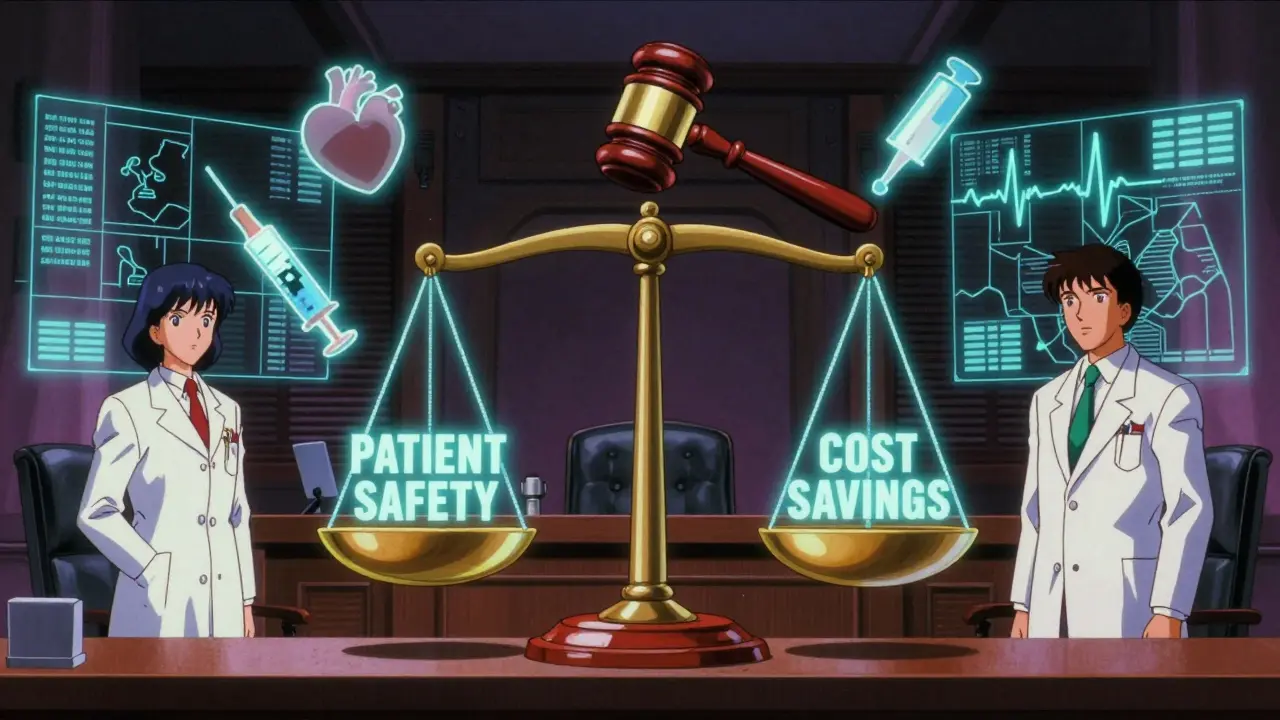
What’s Being Done to Fix This?
The FDA released draft guidance in September 2022 on how to prove therapeutic equivalence for fixed-dose combos. It’s the first time they’ve tried to create a framework specifically for these products. The National Association of Boards of Pharmacy proposed a model law in March 2023 that would create a tiered system:- Simple combos: Two well-established drugs (like metformin + sitagliptin). These could be substituted under clear criteria.
- Complex combos: Novel mechanisms, narrow therapeutic index (like warfarin combos), or multi-drug regimens for cancer or HIV. These require prescriber approval.
What Should Patients and Pharmacists Do Now?
If you’re a patient: Always check your prescription. If your pill looks different, ask: “Is this the same as what my doctor ordered?” Don’t assume substitution is safe just because it’s cheaper. If you’re a pharmacist: Know your state’s rules. Don’t substitute a combo unless you’re certain all active ingredients match the prescription-or you have explicit authorization. Document every decision. When in doubt, call the prescriber. Pharmacists are on the front lines, but they’re not trained to be doctors. The system shouldn’t put them in that position.The Future of Combination Drug Substitution
By 2025, experts predict that 35% of all new drug approvals will be combination products. That’s not a trend-it’s the new normal. Chronic diseases like diabetes, heart failure, and mental health conditions are increasingly treated with multi-drug regimens. The question isn’t whether we’ll see more combos. It’s whether our laws will catch up. Right now, we’re stuck between two bad options: rigid rules that block cost-saving opportunities, or loose rules that risk patient harm. The solution isn’t to ban substitution-it’s to build smart, science-based rules that protect patients while allowing pharmacists to help. Until then, every time a pharmacist hands out a combo pill without knowing if it’s legal, someone’s health is on the line.Can a pharmacist substitute a combination drug without the doctor’s permission?
Generally, no. If the combination drug contains active ingredients not listed on the original prescription, it’s considered therapeutic substitution, which requires prescriber authorization in most states. Pharmacists cannot legally add or swap components without explicit approval, even if the change seems clinically appropriate.
Are combination drugs more expensive than taking separate pills?
Sometimes yes, sometimes no. Brand-name combination drugs often cost more upfront than buying generics separately. But over time, they can reduce overall costs by improving adherence-patients are more likely to take one pill than three. Generic combos are becoming more common and can be significantly cheaper than buying individual drugs.
Why aren’t there more generic combination drugs available?
Regulatory hurdles. The FDA requires full clinical data to prove safety and effectiveness for each combination, even if the individual drugs are already approved. This makes developing generic combos expensive and time-consuming. Between 2015 and 2022, only 37 combination drug products were approved, compared to over 1,200 single-entity drugs.
Do insurance companies encourage substitution of combination drugs?
Yes. Many insurers, including Medicare Part D, push for substitution to lower costs. They may require patients to try a generic combo before approving a brand-name version. But insurers can’t override state laws or force substitutions that violate prescribing rules.
What’s the difference between generic and therapeutic substitution?
Generic substitution means replacing a brand drug with a chemically identical generic version of the same drug. Therapeutic substitution means replacing one drug with a different drug that treats the same condition-like swapping lisinopril for losartan. With combination drugs, therapeutic substitution becomes risky because you’re changing multiple components at once.


Suresh Kumar Govindan
January 25, 2026 AT 23:17The FDA's draft guidance is a charade. They’ve been complicit in this regulatory theater for decades-allowing pharmaceutical conglomerates to engineer patent extensions via combination products while leaving pharmacists as the fall guys. This isn’t about patient safety-it’s about corporate control masquerading as science.
George Rahn
January 27, 2026 AT 13:07Let me be perfectly clear: America’s healthcare system is a feudal fiefdom where bureaucrats in D.C. dictate terms to frontline pharmacists while Big Pharma laughs all the way to the bank. We don’t need more ‘guidance’-we need a revolution in pharmaceutical sovereignty. The patient is not a data point. The pharmacist is not a clerk. And the pill? It is not a commodity to be swapped like a trading card.
Ashley Karanja
January 29, 2026 AT 00:28As someone who’s worked in clinical pharmacy for 18 years, I’ve seen firsthand how the lack of clear substitution protocols creates cascading anxiety-especially for elderly patients managing 8+ meds. The emotional toll is real: patients feel betrayed when their familiar regimen changes without explanation. We need standardized, tiered substitution frameworks that include patient education modules, pharmacist training certifications, and mandatory prescriber-pharmacist communication logs. It’s not just legal-it’s ethical. And yes, I’ve cried in the back room after a 79-year-old asked why her ‘heart pill’ suddenly tasted different. It shouldn’t be this hard.
Karen Droege
January 30, 2026 AT 06:11OH MY GOD. I JUST HAD A PATIENT COME IN YESTERDAY WHO WAS TAKING METFORMIN + JANUVIA SEPARATELY-AND THE PHARMACIST SWAPPED IT FOR A COMBO WITHOUT TELLING HER. SHE HAD A HYPOGLYCEMIC EPISODE BECAUSE THE DOSE RATIO WAS OFF. THIS ISN’T A BUREAUCRATIC NUANCE-IT’S A PUBLIC HEALTH CRISIS. WE NEED A NATIONAL STANDARD. NOW. 🚨💊 #PharmacistsAreNotClerks
Napoleon Huere
January 31, 2026 AT 12:05What if the real question isn’t whether pharmacists can substitute-but whether we’ve outsourced too much of medical judgment to people who aren’t trained to make it? We’ve turned healthcare into a logistical puzzle, and now we’re surprised when the pieces don’t fit. Maybe the answer isn’t more rules-it’s more doctors. More time. More humanity.
Shweta Deshpande
January 31, 2026 AT 20:06I’m from Mumbai, and here we see this all the time-pharmacists handing out combo packs because they’re cheaper and easier to stock. But in India, there’s no real oversight, and people just trust the person behind the counter. I’m so glad you’re talking about this, because in the U.S., at least there’s a framework-even if it’s broken. My mom takes three pills for her heart, and she’d never know if one was swapped unless she checked the label. Please, let’s make this clearer. For everyone.
Aishah Bango
February 2, 2026 AT 08:32This is why you can’t trust pharmacists. They’re not doctors. They’re not supposed to decide what you take. If your doctor wrote three pills, you get three pills. End of story. Any deviation is negligence. And anyone who says ‘it’s clinically beneficial’ is just trying to cut corners while pretending to be a hero. Patients aren’t lab rats.
Simran Kaur
February 2, 2026 AT 21:03As an Indian immigrant in the U.S., I’ve seen both systems. In India, we rely on trust. In America, we rely on paperwork. But neither works perfectly. The real tragedy? The people who suffer most are the ones who can’t afford to ask questions. I hope this sparks change-not just legal, but cultural. Pharmacists should be honored, not burdened. And patients deserve to know, without fear, what’s in their hand.
Neil Thorogood
February 4, 2026 AT 00:44So let me get this straight-pharmacists are legally forbidden from doing the one thing that could save lives and money… but they’re still held liable if something goes wrong? 😂👏 Brilliant. Just brilliant. Next, we’ll ban cashiers from suggesting cheaper brands because ‘it’s not what the receipt says.’ This isn’t healthcare. It’s performance art.
Jessica Knuteson
February 4, 2026 AT 09:25Statistically, therapeutic substitution errors are rare. But when they happen, they’re catastrophic. That’s why the system is designed this way. Risk aversion isn’t bureaucracy-it’s survival. The real problem? The system rewards volume over vigilance. Fix that, and the rest follows.
Robin Van Emous
February 4, 2026 AT 15:39I’m not a doctor. I’m not a pharmacist. But I’ve watched my dad take pills for 15 years. I know what it’s like to see him confused, scared, and exhausted. We need rules that are clear, fair, and human. Not perfect. Not flashy. Just clear. And we need to stop blaming pharmacists for a system that failed them first.
Angie Thompson
February 5, 2026 AT 21:28YES! This is SO important! I work in a clinic and we have patients who cry because they can’t afford their meds-then we find out they’re taking three separate pills because the combo isn’t covered. And the pharmacist won’t swap because they’re scared. It’s heartbreaking. We need a national standard, better insurance coverage for combos, and training for everyone. Let’s make this easier for people who are already struggling. 💙💊 #HealthcareForHumans
Skye Kooyman
February 6, 2026 AT 23:52My pharmacist swapped my combo last month. Didn’t say a word. I didn’t notice until I read the label. I’m fine. But I’m glad someone’s talking about it.