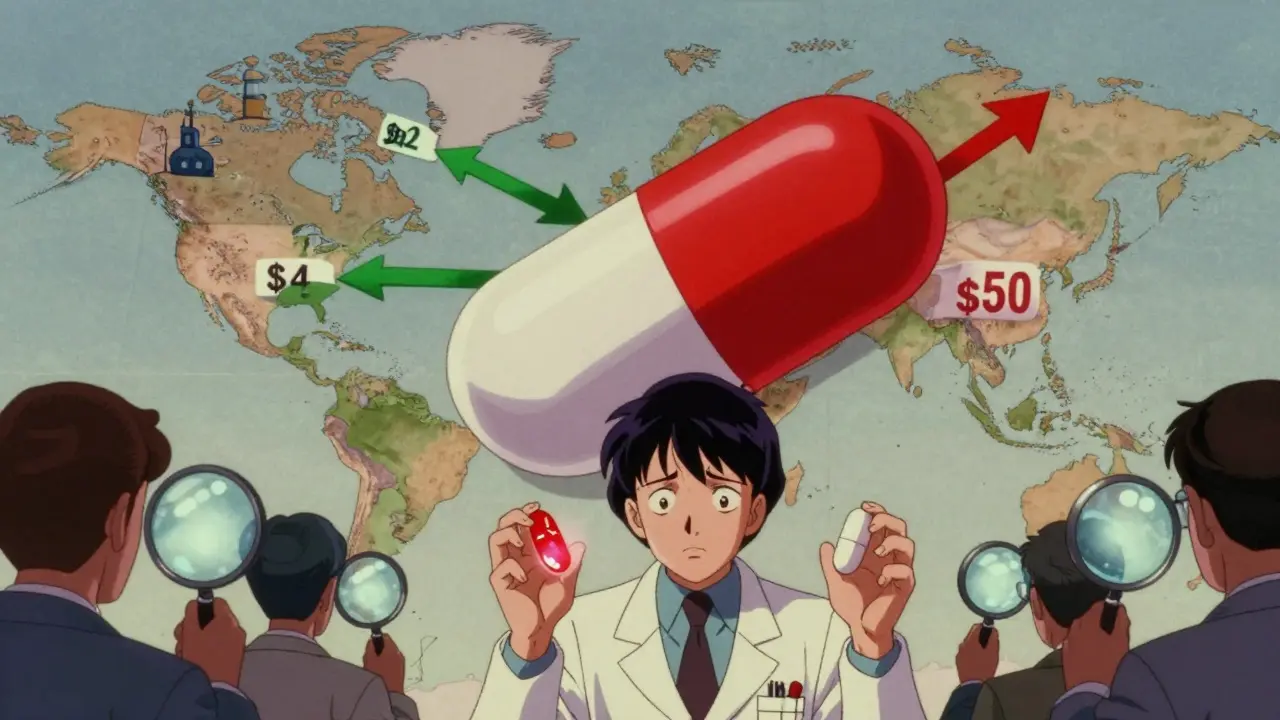
Have you ever filled a prescription for a generic drug in the U.S. and then seen the same pill, in the same dose, sold for a fraction of the price overseas? It’s not a trick. It’s the reality of how generic medicines work across borders. The same active ingredient-say, metformin for diabetes or atorvastatin for cholesterol-can cost $4 in India, $12 in Germany, and $50 in the U.S., even though they’re chemically identical. Why does this happen? And why do some countries have dozens of generic options for a single drug while others barely have one?
How Much of the World Uses Generic Drugs?
Generic drugs make up between 50% and 90% of all prescriptions worldwide, but that number hides a huge gap. In the United Kingdom, 83% of all prescriptions are filled with generics. In Switzerland, it’s just 17%. The U.S. is at 90%, but that doesn’t mean it’s cheap. In fact, Americans pay more for generics than almost any other country. The U.S. spends more on pharmaceuticals per person than any OECD nation, even though most of those drugs are off-patent generics.
This isn’t about quality. It’s about policy, pricing, and culture. Countries like the UK and the Netherlands have government-run systems that push pharmacies to substitute brand-name drugs with generics automatically. In Germany, doctors are required to prescribe generics unless they write "do not substitute" on the prescription. In contrast, in countries like Switzerland and Italy, patients and doctors still trust the original brand names more-even when the generic is proven to work the same way.
Why Do Prices Vary So Wildly?
Price differences aren’t random. They’re built into the system. In the U.S., generic manufacturers can compete, but they don’t always. For some older drugs, only one or two companies make them. When that happens, prices can spike overnight. In 2023, a single generic version of the antibiotic doxycycline jumped from $20 to over $1,800 for a 30-day supply because two of the three manufacturers stopped production. No one else stepped in.
In Europe, governments set price caps. If a generic drug costs too much, the government refuses to pay for it. That forces manufacturers to lower prices just to stay in the market. In India, manufacturing costs are low, competition is fierce, and the government encourages exports. That’s why India makes 40% of the generic drugs used in the U.S.-and sells them for 60-80% less than what Americans pay at the pharmacy.
But here’s the catch: just because a drug is cheap doesn’t mean it’s safe. A 2023 study from Ohio State University found that generic drugs made in India had 54% higher rates of severe side effects-like hospitalizations and even deaths-compared to the same drugs made in the U.S. The issue isn’t the active ingredient. It’s the fillers, the coating, the manufacturing environment. Some Indian factories meet FDA standards. Others don’t. And the FDA doesn’t inspect them all the time.
Who Makes the World’s Generic Drugs?
India is the engine of the global generic market. It produces about 20% of all generic drugs worldwide and supplies 40% of the generics used in the U.S. As of 2023, India had over 750 manufacturing facilities approved by the FDA. That’s more than any other country. Companies like Cipla, Sun Pharma, and Dr. Reddy’s are household names in pharmacies from New York to Nairobi.
But China is catching up. In 2010, China had just 12 FDA-approved drug plants. By 2023, that number jumped to 187. Chinese factories now make everything from painkillers to blood pressure meds. The problem? Quality control is inconsistent. The FDA has shut down several Chinese plants for falsifying data and poor sanitation. Still, because prices are so low, many U.S. pharmacies keep buying from them.
And then there’s the supply chain. During the pandemic, India temporarily stopped exporting 26 key active ingredients. That caused shortages in 22 countries. Antibiotics, heart meds, and antifungals disappeared from shelves. The U.S. had 147 generic drug shortages in 2023 alone. Nearly 70% of them came from manufacturing problems in just one or two foreign plants.
Why Do Some Countries Have More Generic Choices?
It’s not just about manufacturing. It’s about how fast new generics can enter the market. In the U.S., once a patent expires, companies can file to make a generic version. The FDA approves them quickly-but getting pharmacies to stock them takes time. It can take 3 to 6 months before a new generic is widely available.
In the UK, once a drug goes generic, pharmacies are required to dispense the cheapest version unless the doctor says otherwise. That means a new generic hits the market and dominates within weeks. In Switzerland, there’s no such rule. Doctors can keep prescribing the brand-name drug, and insurance pays for it. So even after 10 years, many patients are still taking the original, more expensive version.
The U.S. has more generic manufacturers per drug than most countries-66% of off-patent drugs have two or more makers. But in the UK, it’s only 50%. That’s because U.S. companies are more aggressive about entering the market, even if it means cutting prices to the bone. In Canada and Germany, fewer companies compete, so prices stay higher.
What About Quality and Safety?
Everyone assumes generics are the same. They’re not. The FDA requires generics to be bioequivalent-meaning they release the same amount of active ingredient into the bloodstream within 80-125% of the brand-name drug. That sounds strict. But that 45% range is wide. Two generics can be at opposite ends of that range and still both be approved.
Some patients notice the difference. On Reddit, users report that switching from a U.S.-made generic levothyroxine to an Indian-made version caused fatigue, weight gain, and heart palpitations. The active ingredient is identical. But the fillers-lactose, dyes, binders-can affect how the drug is absorbed. For drugs with narrow therapeutic windows-like thyroid meds, blood thinners, or seizure drugs-those tiny differences matter.
And inspections? The FDA gives foreign manufacturers advance notice before inspections. That means factories can clean up, fix problems, and hide issues. In the U.S., inspections are unannounced. So a plant that passes an FDA inspection in India might not pass if it were inspected without warning.

What’s Changing Now?
The U.S. Inflation Reduction Act of 2022 is trying to fix some of this. It gives the FDA more money to inspect foreign plants and speeds up approval for complex generics like inhalers and creams. The European Union is pushing for a unified policy-aiming for 80% generic use by 2030. The WHO is creating a global benchmark tool to standardize quality checks.
But the biggest challenge? Patent tricks. Drug companies file dozens of minor patents on the same drug-changing the shape, the coating, the release time-to delay generics. Between 2015 and 2022, 1,247 such patents were filed on just 12 top-selling drugs. That’s how a drug that should have gone generic in 2018 is still under patent protection in 2025.
Meanwhile, AI is starting to help. Companies are using machine learning to design generics faster-cutting development time from 5 years to under 2. If regulators can keep up, this could bring down prices globally. But only if quality stays high.
What This Means for You
If you’re on a generic drug, know this: your pill might have been made in India, China, or the U.S. The label won’t tell you. If you notice side effects after switching brands, talk to your pharmacist. Ask if the manufacturer changed. If you’re traveling, don’t assume your U.S. generic will be available-or safe-overseas. And if you’re buying online from a foreign pharmacy, check if it’s certified by PharmacyChecker or similar groups.
There’s no single answer to why generics vary so much. It’s a mix of politics, profit, and regulation. But understanding it helps you make smarter choices-whether you’re paying out of pocket, on insurance, or just trying to stay healthy.
Why are generic drugs cheaper than brand-name drugs?
Generic drugs are cheaper because they don’t need to repeat expensive clinical trials. Once a brand-name drug’s patent expires, other companies can copy the active ingredient and prove it works the same way through simpler bioequivalence tests. They also spend far less on marketing. That’s why a generic version of Lipitor can cost 90% less than the original.
Are generic drugs as safe as brand-name drugs?
Yes-when they’re made under proper standards. The FDA and EMA require generics to meet strict bioequivalence rules. But safety depends on manufacturing quality. Some foreign factories have poor sanitation or falsify data. A 2023 study found Indian-made generics had 54% higher rates of severe side effects compared to U.S.-made versions. Always report unexpected reactions to your doctor.
Why can’t I get the same generic drug everywhere?
Different countries approve different manufacturers. A generic made by Sun Pharma in India might be approved in the U.S. but not in Germany. Also, some countries have rules that limit which generics pharmacies can stock. In Switzerland, doctors often prescribe brand names, so pharmacies don’t carry many generics. In the U.S., pharmacies stock the cheapest option, so you might get a different maker each time.
Can I buy generic drugs from other countries to save money?
You can, but it’s risky. Online pharmacies in Canada or India often sell the same drug for 60-80% less. But the FDA doesn’t regulate them. Some are legitimate. Others sell counterfeit or substandard drugs. Use only certified sites like PharmacyChecker. Never buy from unknown sellers. And always tell your doctor if you switch sources.
Why do some countries have more generic options than others?
It’s about policy. Countries with mandatory substitution-like the UK and Netherlands-encourage multiple manufacturers to enter the market quickly. In the U.S., competition is high, but price spikes happen when only one or two companies make a drug. In Switzerland and Italy, doctors and patients prefer brand names, so generics never gain traction. Regulation, reimbursement rules, and cultural trust all play a role.
What’s the future of global generic drug availability?
AI and better manufacturing tech could cut development time for generics from 5 years to 2. The U.S. and EU are pushing for faster approvals and stronger inspections. But patent evergreening and regulatory fragmentation still block progress. If countries harmonize quality standards and stop delaying generics with fake patents, prices could drop worldwide. Until then, disparities will remain.
If you’re managing a chronic condition, don’t assume your generic is the same everywhere. Keep track of the manufacturer name on your bottle. Ask your pharmacist if anything changed. And remember: cheaper doesn’t always mean better-especially when your health is on the line.







Brooks Beveridge
December 18, 2025 AT 08:03It's wild how the same pill can cost $50 here and $4 in India. I get it-profit margins, patents, supply chains-but it feels like our system is broken when your life depends on something that shouldn't be this expensive. We're not talking luxury goods here. We're talking insulin, metformin, blood pressure meds. People are choosing between meds and rent. And we act surprised when someone gets sicker?
It’s not just about price. It’s about dignity. And we’ve let corporations turn healthcare into a game of Monopoly with real human lives as tokens.
Anu radha
December 19, 2025 AT 05:44India makes so many pills for you. We work hard. But some factories get bad press. Not all bad. Many are clean. Many help the world. Please don’t think all Indian drugs are dangerous. My uncle takes generic from India. He is fine. He is happy. We trust our medicine.
Victoria Rogers
December 21, 2025 AT 01:26Oh wow, so now we’re blaming India for our broken healthcare system? Let me guess-next you’ll say the FDA is lazy because they don’t inspect every single factory in real-time. Newsflash: the U.S. government lets Big Pharma write the rules. The real problem isn’t foreign factories-it’s that our regulators are owned by the same companies that profit from inflated prices. You want cheap generics? Break the patents. Don’t scapegoat the Global South.
Meghan O'Shaughnessy
December 22, 2025 AT 03:05It’s funny how Americans act like they invented medicine. Meanwhile, 80% of the world’s generic pills come from places we’ve spent decades calling "third-world". We import the drugs, then act like we’re the only ones who know how to make them safe. Meanwhile, our own pharmacies can’t even tell you where your pill came from. The label just says "generic". That’s not transparency. That’s ignorance dressed up as convenience.
Kaylee Esdale
December 22, 2025 AT 11:26generic drugs are just pills right? same stuff right? but then you switch and feel weird and your doc says "it's all the same" and you're like... ok but my brain feels like it's wrapped in wet socks now. why? because the filler stuff? the dye? the coating? it's not magic. it's chemistry. and we treat it like it's not. dumb.
CAROL MUTISO
December 23, 2025 AT 06:40Let’s be real-the U.S. pays more for generics because we’re the world’s pharmacy subsidy program. Other countries negotiate prices. We let middlemen and PBMs gouge us. Meanwhile, India and China are quietly building the infrastructure to dominate the future of medicine. We’re stuck in a loop of patent trolling and corporate lobbying while the rest of the world moves on.
And don’t get me started on the "FDA inspections with advance notice" thing. That’s like having a health inspector show up right after you clean the kitchen. Of course it looks clean. That’s not safety. That’s theater.
Erik J
December 24, 2025 AT 11:57Why does the FDA allow such a wide bioequivalence range? 80-125%? That’s a 45% swing. Two pills can be at opposite ends of that and still be "the same." For thyroid meds, that’s not a difference-it’s a risk. I switched generics once and my TSH went from 2.1 to 7.8. No change in dosage. Just a different manufacturer. Took months to stabilize. No one warned me.
BETH VON KAUFFMANN
December 24, 2025 AT 23:58Let’s cut through the noise. The real issue isn’t manufacturing-it’s reimbursement structures. In the U.S., PBMs get kickbacks from brand-name manufacturers to steer patients away from generics. That’s why even after patent expiry, some drugs stay expensive. It’s not about cost. It’s about kickbacks. The FDA can inspect factories till they’re blue in the face, but if the system is rigged, nothing changes. We’re treating symptoms, not the disease.
Martin Spedding
December 26, 2025 AT 07:40U.S. generic prices? A joke. I lived in London for 3 years. Got my statins for £2 a month. Came back here? $50. Same pill. Same company. Same batch code. Just different country. We’re not broken. We’re greedy. And we call it capitalism.
Donna Packard
December 26, 2025 AT 07:59It’s easy to feel hopeless about this stuff. But small actions matter. Ask your pharmacist who makes your meds. Switch to a different brand if you feel off. Join patient advocacy groups. We’re not powerless. We just got used to being silent.
Patrick A. Ck. Trip
December 26, 2025 AT 20:09While I acknowledge the structural inequities inherent in global pharmaceutical distribution, I must emphasize that the commodification of life-saving therapeutics represents a profound moral failure of neoliberal governance structures. The dissonance between bioequivalence metrics and clinical outcomes underscores a systemic epistemological rupture in regulatory epistemology. We must recalibrate our ontological frameworks to prioritize patient-centered pharmacovigilance over market-driven efficiency paradigms.
Chris Van Horn
December 28, 2025 AT 20:04Oh, so now it's India's fault that Americans are dumb enough to pay $50 for a pill? We import 80% of our active ingredients from China and India, then act shocked when the quality varies? We built this mess ourselves. We outsource production, then complain when the product isn't perfect. Meanwhile, our politicians take millions from pharma lobbyists to block price controls. Don't blame the factories. Blame the people who let them be exploited.
Virginia Seitz
December 30, 2025 AT 08:01My grandma takes Indian metformin. She’s 82. Still walks 3 miles a day. 😊
USA generics made her dizzy. Indian one? She feels better. So… maybe the problem isn’t the country. Maybe it’s the system.
Steven Lavoie
December 30, 2025 AT 21:21My cousin works in a generic drug plant in Gujarat. He told me they have to meet FDA standards to export. But the inspections are scheduled weeks in advance. So they clean up, fix the leaks, hide the dust. Then it’s business as usual. The system is designed to look good on paper, not to keep people safe. That’s not negligence. That’s policy.
Michael Whitaker
December 31, 2025 AT 04:41Let me just say this: if you’re buying drugs from random websites because you can’t afford your prescription, you’re not being clever-you’re being desperate. And that’s not a market failure. That’s a moral failure. The fact that this is even a conversation we’re having in 2025 should shame us all.